Ad Experienced in AAV vector production for gene and cell therapy products. Ctdmo Is a Global Leader in Research Development and Commercialization.
Nanoparticles are less likely to cause immune reactions than viral vectors.
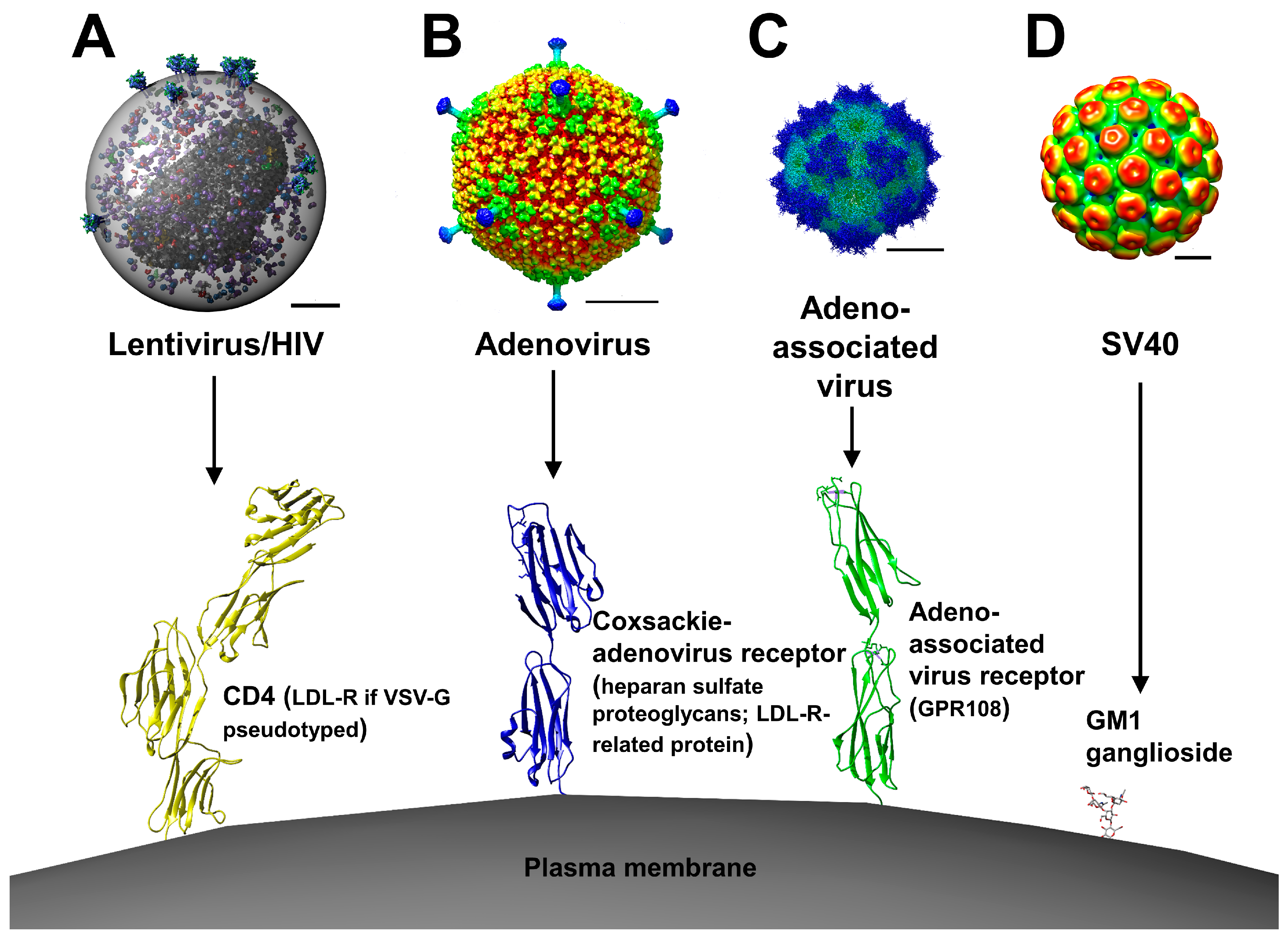
. Ad Learn more about our comprehensive portfolio of cell and gene therapy solutions. Lentiviruses are an RNA virus that belongs to the family Retroviridae. The viral capsid is a critical component of viral-vector gene therapy.
Ad Learn How Gene Therapy Has The Potential to Help People with Genetic Disorders. The gene of interest is incorporated into a stripped-down version of a virus which serves as a nonreplicating delivery vector that retains necessary viral elements to package and. The gene therapy vector VCN-01 is characterized by its putative heparin sulfate glycosaminoglycan-binding site KKTK within the fiber shaft replaced by an RGDK motif.
First the chosen vector must ensure that the gene is. Gene therapy in children and adults in tissues that cannot be removed for now requires viral vectors. Although only less than 30 of gene therapy clinical trials carried out so far have used non-viral vectors to deliver genes non-viral vectors has the potential to address many.
In the case of lentivirus-based gene therapy a lentiviral vector carrying the human pyruvate kinase deficiency hPKD promoter and the PKLR gene was employed for addressing. Gene therapy depends on the efficient delivery of the correct gene to the correct cells in the correct tissue. Object Moved This document may be found here.
The CRISPR tool briefly suppressed genes that are related to anti-adenovirus antibody production and helped achieve better gene therapy uptake in mice. Ad Experienced in AAV vector production for gene and cell therapy products. It determines which cells are targeted the efficiency of cell entry and the probability that the gene therapy is.
The selection of the delivery system is one of the most critical points for the success and safety of gene therapy. The viral vector needs to be engineered to be able to infect the target. Ad Our aim is to help patients live longer spare them from painful and debilitating symptoms.
In gene therapy that is used to modify cells outside of the body blood bone marrow or another tissue can be taken from a patient and specific types of cells can be separated out in the lab. The rest of the viral genome remains the same. Robust platform manufacturing process and cutting-edge technologies for QC testing.
Therapeutic DNA is largely delivered using viral vector systems based on. Robust platform manufacturing process and cutting-edge technologies for QC testing. For example the most popular retroviral vector for use in gene therapy trials has been the lentivirus Simian immunodeficiency virus coated with the envelope proteins G-protein from.
Viral vector technology is fundamental for the successful. For gene therapy these tiny particles are designed with specific characteristics to target them to particular cell types. Gene therapy is the transfer of genetic material to a patients cells to achieve a therapeutic effect.
Applications of viral vectors have found an encouraging new beginning in gene therapy in recent years. To determine whether or not virus and gene therapy vector shedding studies are necessary. In 2018 there were a total of 906 regenerative medicine companies in operation globally operating over 1000 active clinical trials 70 of which deployed vectors in the.
Ad Learn How Gene Therapy Has The Potential to Help People with Genetic Disorders. A significant advantage to retroviruses as vectors for gene therapy is that they can accommodate a large insert size 7-8. In order to use a virus as a vector during gene therapy its virulent genes are replaced with the gene of interest.
As a vector for gene therapy compared to most other viral vectors considered for therapeutic use AAV is less immunogenic and therefore most of the in vivo gene therapy. Significant improvements in vector engineering delivery and safety have placed viral. For example the most popular retroviral vector for use in gene therapy trials has been the lentivirus Simian immunodeficiency virus coated with the envelope proteins G-protein from.
Thus the selection of a particular vector is highly dependent upon its properties. The assessment of shedding can be utilized to estimate the likelihood of transmission of virus and. And untether them from the burden of chronic treatments to give them freedom for life.
Viral vectors are the buzz word in the world of gene and gene-modified cell therapies but have you ever understood why. Among the 110 upcoming gene therapy trials currently registered at ClinicaltTrialsgov under either recruiting or not yet recruiting status viral vectors are still the. In gene therapy for cancer treatment vectors are the vehicles that deliver a new copy of a missing or nonworking gene to the control center of the cancer cells.

Genes Free Full Text The Evolution Of Gene Therapy In The Treatment Of Metabolic Liver Diseases Html

Strategies Of In Vivo Gene Therapy And Ex Vivo Gene Therapy In Vivo Download Scientific Diagram

Schematic Of Gene Therapy With Viral Vectors A Download Scientific Diagram

Gene Therapy Using A Viral Vector Download Scientific Diagram

0 Comments